- HOME
- Research Content
- Li Zhenghua
Research Content
Project Assistant Professor Li Zhenghua
FAP group
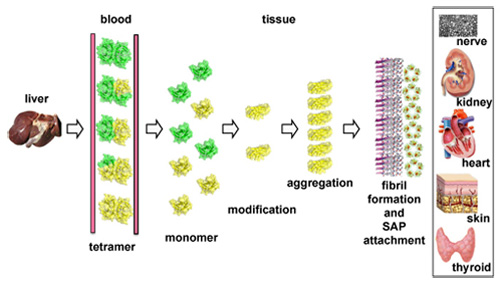
Familial amyloidotic polyneuropathy is an autosomal dominant disease characterized by peripheral and autonomic polyneuropathy. Average age at onset is at around 30’s to 40’s. Once the disease begins, it is progressive and fatal within 10 to 20 years. FAP is caused by the point mutation of transthyretin (TTR) gene and mutant proteins are deposited as amyloid in various tissues including peripheral and autonomic nervous tissue, heart, and kidney. Human TTR gene spans about 7 kilobases (kb) and contains four exons and three introns. TTR is synthesized mainly in the liver, choroid plexus and retinal pigment cells, and is secreted into plasma, cerebrospinal fluid, and vitreous body, respectively. It circulates as a tetramer (55 kDa) composed of four identical, non-covalently associated subunits and serves as a transport molecule for thyroxine and the retinol binding protein (RBP). Among 110 TTR variants, more than 90 of these are associated with human amyloidosis. Amyloid deposition processes are now summarized as follows (see figure), (1) expression of TTR in liver, (2) excretion of TTR tetramer into serum, (3) dissociation of TTR tetramers to monomers, (4) modification of TTR monomer, (5) aggregation of monomers, (6) amyloid fibril deposition and attachment of serum amyloid P component (Apcs) to fibril.
To gain insight into the pathogenesis of TTR-associated amyloidosis (ATTR), we have generated many transgenic/knockout/knockin mouse lines that carry mutant TTR genes or other related genes such as serum amyloid P component (Apcs) gene. Using these mice, we revealed following things, (1) the 6kb upstream region of human TTR gene contains cis-regulatory elements necessary for correct temporal and spatial expression of TTR gene, (2) amyloid deposition starts at around 6 months of age although the serum level of TTR reaches at adult level at 4 weeks of age, (3) amyloid deposition is not observed in transgenic mice when kept under SPF conditions, suggesting that environmental factors are involved in the onset of amyloid deposition(6), (4) Cys10 plays an important role for amyloid deposition through conformational change, (5) serum amyloid P component (Apcs) does not play active role in triggering of amyloid deposition, (6) mouse TTR molecules stabilize human/mouse hybrid TTR tetramers(5), (7) human TTR tetramers have low binding affinity to mouse retinol binding protein 4 (RBP4) (5).
Based on above findings, we think it important to create an ideal mouse model for FAP, that is, humanized mice at mouse TTR and RBP4 loci to analyze pathologic processes of disease development and to develop new way of treatment. We first produced the null mutant mice for mouse Ttr locus using a targeting vector which contains a neomycin resistance gene flanked by lox71 and loxP. Then, the neomycin resistance gene is replaced with a human TTR cDNA by Cre-mediated recombination. We succeeded to establish the mouse lines carrying a human normal (Val30) or mutant (Met30) TTR cDNA at mouse Ttr locus. The introduced human TTR cDNA is expressed quantitatively and qualitatively similar manner to that of mouse endogenous Ttr gene in the absence of mouse TTR(5). Furthermore, we established the mouse line carrying the human RBP4 cDNA at mouse Rbp4 locus. These humanized mice are mated to produce double humanized mice at both Ttr and Rbp4 loci. In collaboration with a pharmaceutical company, we are now analyzing whether drugs can prevent amyloid deposition in these humanized mice.